By Denis Thomas
God commended the people of Berea because they searched the Word to verify if what they were hearing was true (Acts 17:11-12). There is nothing wrong with people questioning claims made by others. In fact, God instructs us to prove all things, hold fast that which is good. I am not a chemist, but I can question claims made which challenge the accuracy of God’s Word. In fact, Radiometric Dating is one of the methods used to challenge the truth of Biblical creation.
For 31 years, I was an electrician working in the laboratories and production facilities at Savannah River Site (South Carolina), one of the US government’s nuclear facilities, when I became interested in creation science after hearing Ken Ham speak in 1990. My eyes were opened as I wrote a critique of my son’s eighth grade science text in 1993 and contended with the school and school board.
Since retirement, I have taken a couple of chemistry courses and studied mass spectrometry. Although I am not an “expert” in the field, I recognize some of the limitations of the instrument which invalidate the claims of radiometric dating. Hopefully, this article which is a result of my self-study will cause people to look at the limitations of the mass spectrometer, the instrument which measures the mass to charge ratios, m/z, for the purpose of radiometric dating. I approach the subject with 1 Thessalonians 5:19-22 in mind.
There are 3,184 isotopes, variations of the 118 elements. Most of these are man-made. The radiometric dating we are concerned with here is the measurement of naturally occurring radioactive isotopes and their daughter isotopes. We are also limiting the discussion here to potassium-argon dating, which is one of the more popular dating methods. Of the 24 isotopes of potassium (K), only three occur naturally: 39K, 40K, & 41K. (The number prior to the symbol represents the total protons and neutrons within that isotope.) Natural abundances of these are as follows: 39K – 93.25810%; 40K – 0.01170%; and 41K – 6.73020%. 39K & 41K are stable, while 40K has a half-life of 1.2519×109 years. When 40K decays, 89.28% forms 40Ca (calcium), with the remaining 10.72% becoming 40Ar (argon). The natural abundance of 40Ar is 99.60030% and makes up 0.93% of the air we breathe.
Of the 580 minerals which contain potassium in its empirical formula, for simplicity we are focusing on hieratite, K2SiF6, a white crystal found in Pennsylvania and in Italy. Of the 18 isotopes of fluorine, only stable 19F occurs naturally. There are 23 isotopes of silicon with 3 stable isotopes naturally occurring: 28Si, 29Si, & 30Si. Their natural abundances are: 92.22970%, 4.68320%, & 3.08720% respectively.
Avogadro’s number has been refined by the International Bureau of Weights and Measures, BIPM, as the exact value 6.02214076 x 1023, effective May 2019. This large number represents an estimate of the number of molecules in a mole of a substance. It can also represent the number of atoms in a mole of an element. It testifies of how amazing our Creator God Almighty is, to make the universe out of such small building blocks, which are made up of even smaller particles: electrons, neutrons, & protons!
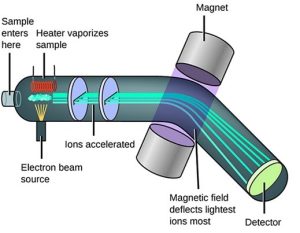
There are various types of mass spectrometers (MS). Most contain a detector, an electromagnetic device, a filter, a vacuum pump, an ionizer, and a heater. The heater is used to convert the sample into a gas. The vacuum pump is used to remove outside air. For the detector to detect the particle, it must have an electric charge, which is accomplished through ionization. There are numerous types of ionizing methods today, but here we will only focus on electron ionization, EI, where electrons flow from a cathode plate to an anode, randomly splitting the sample molecules. While most of the ions created with EI will cause an electron to be ejected from the molecule and randomly splitting the molecule, sometimes the molecule can capture an electron to gain a negative charge, becoming an anion. The electrons are pulsed, about 1 microsecond duration per second. The detector can only measure the intensity of the charge, while the strength of the magnetic field used to deflect the charge determines the m/z ratio. Heavier ions require a stronger field to deflect the ion to the detector. When the magnetic field is set for a particular mass weight, heavier and lighter ions will not be deflected to the detector and will be electrically discharged in the filter and will become like the uncharged portion of the molecule which does not get counted. For potassium-argon dating, most of the isotope we are concerned with will pass through undetected.
Figure 1-Basic Principle of a Mass Spectrometer
“The usefulness of electron ionization decreases significantly for compounds above a molecular weight of 400 Da.”[i] (Da = Daltons.) Calibration of the magnetic field must be accurate. This can be tested by analyzing a controlled gas. In one of the labs I worked at, carbon-14 was used to check MS calibration.
Figure 2-A portion of the controls of a Quadruple Mass Spectrometer
There are several types of filters. The quadrupole has four metal rods. An AC, alternating current/voltage, is applied to the rods, which can be offset with a DC voltage. It may also include an ion trap. The quadrupole MS is more economical than other types of MS andis simpler to learn. Resolution is controlled by adjusting the DC voltage. Too high of a resolution and a significant portion of the ions oscillate far enough to become lost.
The mass spectrometer can process up to 15,000 of the lighter charged particles, whether cation or anion, per second. A cation has a positive charge because it is missing one or more electrons, while an anion has a negative charge. The majority of ions created with electron ionization will be cations. Most will have a +1 charge (-e), while some can be +2 (-2e). A mass of 40 with a +2 charge would be detected as 20 m/z. Ionization of biochemical materials, which can range up to 25,000 Daltons, can have a charge of up to ±15 or slightly more. While a run is typically four minutes with the ability to combine the results of four runs, a run for the heavier biochemical analysis can last eighty minutes.
A controlled gas such as helium, nitrogen, or argon can be supplied to the unit at up to 6 BAR, about 80 psi. These are referred to as background ions. Although they can be subtracted from the result by the computer, part of the count of 15,000 cps is counting these background ions which means a smaller percentage of the sample is being counted in a run. “Subtracting the background ions may make it easier to identify what masses are attributed to your sample, versus those attributed to the solvents” (from page 44 of Thermofisher MSQ operator’s guide).
With knowledge of Avogadro’s number, we can get a better understanding of how small the sample size used for dating is. An average dried fingerprint weighs 4 micrograms, or 0.000004 gram. Hieratite weighs 220.27 grams/mole. To determine how many average dried fingerprints it would take to equal the weight of one mole of hieratite we divide 220.27 by 0.000004 and find it equals 55,067,500. To determine the approximate number of molecules of hieratite which equals the weight of one dried fingerprint, divide Avogadro’s number, 6.02214076 x 1023 by 55,067,500 = 10,935,925,473,282,789. Now let’s divide that by the MS count rate of 15,000 molecules per second = 729,061,698,219; then by 86,400 seconds in a day = 8,438,214 and divide this by 365.24 days per year = 23,103.2 years to process the molecules of hieratite equaling the weight of one dried fingerprint if no solvents or background gas was also being counted. Our count is extending for 16 minutes.
The sample size for radiometric dating is between 100-500 picograms, which is less than 1/8000th that of a dried fingerprint. Even with such a small sample size, the majority of the molecules do not get counted. For all of the molecules of a 500 picogram sample to be counted by MS would take over 2.5 years. One source of error is the potential for contamination, whether by exposure to air, by handling, or by carryover which would be molecules left in the instrument from a previous run. One limitation is the small sample size.
While the instrument can quantitate and display a graph of m/z ratios, it is up to the operator to interpret what potential isotopes can equal a m/z charge. This may have been done by someone else and incorporated into a computer program. Since Hieratite contains six fluorine atoms, one silicon atom, and two potassium atoms, we could make a chart of possible m/s ratios or use Excel to calculate possible ratios as I did. Of cations with a +1 charge, there are 99 possible m/z ratios ranging from 19 to 226. 74 of these may contain 40K, but only 5 would definitely contain 40K. If we also consider ions with a +2 charge, there are 180 m/z possibilities with 46 having a +2 charge and 10 definitely containing 40K. If a solvent is used, it would increase the possible m/z combinations and reduce the amount of the sample being detected. In this example, the possibility of other types of atoms have been excluded.
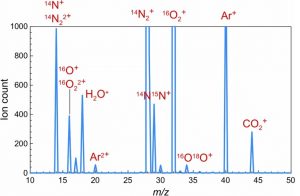
Figure 3 is an m/z clipart of air. The +2 charge of Argon is identified, as well as the +2 charge of oxygen and nitrogen combining with individual atoms of O & N. Note the combination of 16O18O, and 14N, 15N. Unless the quantities of m/z rise notably above the baseline, they are not identifiable. The spikes at 17 & 30 are not labeled. Looking at the database of isotopes, the spike at 17 could be 17O, or hydroxide 16O1H, or 4He + 13C, or several less likely combinations.
Figure 3-m/z Analysis of Air
Although the instrument can process up to 15,000 cations (or anions) per second, it can only analyze the spectrum of up to 20 m/z per second. Our spectrum ranges from 19 to 226. The natural abundance of 40K is 0.01170%. During four 4-minute runs, about 14,400,000 cations resulting from ionization of hieratite would be measured with about 3,370 atoms of 40K or its daughters. Since the uncharged portion does not get counted, it is safe to realize that part of the 40K & 40Ar we are looking for passes uncharged and undetected. Part of the 40K may or may not be in the 69 of 74 m/z ratios which may or may not contain 40K. As the MS is only examining up to 20 of the m/z spectra per second, the intensity of the magnetic field is such that the other cations are being electrically discharged by the filter. A large portion of the 40K will never make it to the detector.
Wait! The mass weights of 40Ca, 40Ar, & 40K are virtually identical. A mass spectrometer cannot distinguish between these. That takes us back to operator interpretation and/or deception.
Let’s pretend for a moment that the instrument could make the distinction between 40K and 40Ar. Argon is an inert gas. Because argon contains eight electrons in its outer orbit, it is resistant to bonding with other elements, such that there are no known compounds containing Argon at normal temperatures. To claim potassium to argon dating, it must be assumed that once the potassium decays into argon that the argon is trapped by surrounding molecules and cannot escape. This is referred to as a closed system. Such assumption is also made with the uranium to lead dating method.
If the sample is first dissolved or crushed in a sealed vial, then the argon would escape, and a larger quantity would get deposited onto the needle used to insert sample into the MS instrument. That would greatly increase the age calculation. Look at the m/z chart for air (Figure 3) and notice the argon. If the sample has been exposed to air which has an abundance of 0.93%argon, it would appear the sample has a larger quantity of 40K, 40Ca, or 40Ar than reality.
As with any statistical analysis, greater accuracy is accomplished with larger sampler sizes. Since the majority of 40K decay produces 40Ca, the logical choice of measurement would be a potassium-to-calcium dating. However, that would assume that no calcium was in the sample when it was formed. Assumption of none of the daughter elements present when the sample is formed is made with the majority of radiometric dating methods. While a large amount of hieratite is comprised of potassium, the natural abundance of 40K is so small that even if there was an instrument with the count rate of the MS that could distinguish between 40Ar, 40K and 40Ca, the interpretation would likely not even be in the ballpark of accuracy.
Solubility is another limitation of MS. If solubility is poor, it will be difficult to ionize or vaporize the sample. Purity is another factor. There are several types of detectors, each with advantages and disadvantages. I am certain there are other limitations not mentioned, but what is mentioned is enough to invalidate potassium-argon dating.
In conclusion I ask the reader of this article to be like a Berean and understand that I believe that my research shows that the current standard methods using mass spectrometers for dating rocks have tremendous inherent limitations. These limitations consistently result in vastly incorrect dates.
As with much of false science, follow the money or social agenda. Numerous times in scripture, God warns the believer against being deceived.
[i] Mass Spectrometry for Biotechnology, Gary Siuzdak, ©1996, page 7