Radiocarbon dating, also called carbon-14 dating, is a straightforward method for estimating the age of fossils, wood, seashells, and other things that were once living. The idea is straightforward, but its application is not. This is because of all the factors that enter into any application of the method. I hope to explain the method and its difficulties in this article.
Carbon-14 dating is a radiometric dating method, but cannot be used for dating rocks. It can only be used for organic (animal or plant) materials and cannot theoretically be used for anything older than 100,000 years because in that amount of time all the radioactivity would be spent. The half-life of carbon-14 is 5,730 years, compared with millions or billions of years for the radioisotopes used in other common radiometric dating methods.
HOW DOES THE CARBON-14 FORM?
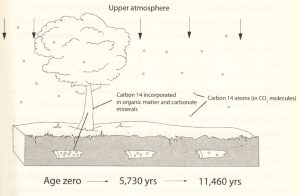
In figure 1 is a diagram of how most carbon-14 is formed and transmitted throughout the biosphere. It forms high in the upper atmosphere. Cosmic rays knock neutrons out of atomic nuclei. These displaced neutrons move at high speed and collide with ordinary nitrogen (N-14) atoms at lower altitudes, converting them into carbon-14. Unlike common carbon-12, carbon-14 is unstable (or radioactive), and it slowly decays back to nitrogen.
These carbon-14 atoms drift downward toward the surface of the earth and combine with oxygen to make carbon dioxide CO₂. As the plants and trees absorb the CO₂ the carbon-14 atoms become part of their fiber. When animals (and humans) take in the plant fiber it is used in their carbon makeup. Finally, after death the C-14 decays and is not replaced.
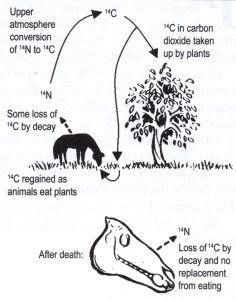
Figure 2 is another way to understand the process. All plants take in carbon, so all are radioactive to a very small degree. This type of radioactivity is not harmful to life. The ratio of C-12 to C-14 atoms is about a trillion to one! One estimate is that the total weight of C-14 atoms formed each year in the earth’s atmosphere is only 21 pounds.
HOW IS THIS PROCESS USED AS A CLOCK?
Carbon-14 spontaneously decays back to nitrogen at a measurable rate. Each 5,730 years half of the radioactive carbon atom will decay into nitrogen. The science is that measuring the amount remaining in a dead specimen allows for the length of time since death to be calculated. That result is the time since the organism stopped replacing C-14 by eating or absorbing.
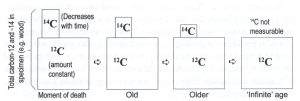
Figure 3 illustrates the process showing the C-12 constant amount and the C-14 decreasing amount with time. This method was introduced in 1952 by University of Chicago scientist Willard Libby. He received a Nobel Prize in Chemistry in 1960 for proposing this dating technique. The half-life segments go like this: 5,730 years to 11,460 years to 17,190 years to 22,920 years to 28,650 years and so forth. This is why after 100,000 years most of the C-14 has decayed and the method can no longer be used. These years are all evolutionary supposed deep time years past the 4,500 years the time since the global Flood at the time of Noah.
SO, WHAT ARE THE PROBLEMS?
First Problem: In 1952, when Willard Libby proposed the radiocarbon dating technique, he called attention to the critical assumption that the ratio of carbon-14 to carbon-12 has been constant. Another way to say this is that the ratio was always in equilibrium. But was that true?
Libby tested the assumption by making various measurements and calculating how rapidly the carbon-14 was forming and decaying. Surprisingly, he found that carbon-14 was entering the atmosphere faster than it was decaying. That meant that there was no ratio equilibrium and there was much less atmospheric carbon-14 in the past. It also meant that the conclusion that the lack of carbon-14 in dead animals and plants was not strictly due to the time that had passed due to the decay of the carbon-14. But since Libby believed in an old earth that lacked any global Flood, he concluded that equilibrium could be assumed in the technique.
More accurate measurements later determined that radiocarbon is forming 28-37% faster than it is decaying. That means that the farther one looks back in time, the greater the out-of-balance condition would have been until the time of the global Flood. [See Melvin Cook, “Nonequilibrium Radiocarbon Dating Substantiated,” Proceedings of the First International Conference on Creationism, Vol. 2, 1986, pp. 59-68.]
The Flood would have greatly upset the carbon balance due to the rapid burial of a huge amount of carbon that was in the pre-flood forests and other vegetation. This all became coal and oil and lowered the total carbon-12 in the biosphere. This and other effects of the Flood would, unless corrected for, give ages much older than the true ages.
Also, volcanoes emit much CO₂, depleted in carbon-14. Since the Flood was accompanied by much volcanism according to creationist models, fossils formed early in the post-flood period would also give ages older than they really are.
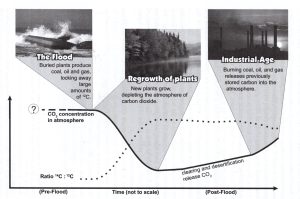
Figure 4
Figure 4 illustrates the likely effect of the Flood and man’s activities on carbon isotopes which affect carbon dating.
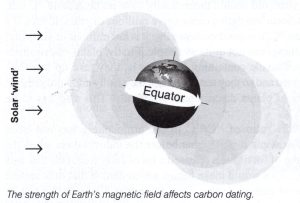
Second Problem: The number of cosmic rays hitting earth’s upper atmosphere affects the amount of carbon-14 produced and therefore would affect the accuracy of the dating system. The primary factors affecting the number of cosmic rays coming to the earth vary with two things. One is the activity of the sun, and the other is the location of the earth with respect to magnetic clouds in the Milky Way galaxy as the solar system moves through them. It is a well-known fact that the strength of the earth’s magnetic field that protects the biosphere from cosmic rays has been steadily decreasing. Therefore, more carbon-14 is being produced now than ever before.
Third Problem: Since the development of radiocarbon dating methods more precise equipment has been developed that can measure a very small amount of carbon-14 in a sample. Blind tests of coal and diamond samples have demonstrated that there is still carbon-14 remaining in them. These samples were said to be millions or billions of years old according to the evolutionary paradigm. The fact that they still have carbon-14 in them is another strong argument against deep time.
Dr. Walter Brown says that “radiocarbon ages less than 3,500 years old are probably accurate.” [See the website for the Center for Scientific Creation.] However, it is interesting to note that archaeologists still prefer dates determined from historical records over any radiocarbon dated method. Perhaps someday radiocarbon dates will be accurately corrected and correlated to the biblical timeline. Until then, radiocarbon dating methods should be used and accepted with great caution.
J.D. Mitchell
Figures sources:
Figure number 1 from DeYoung, Don, Thousands…Not Billions, Master Books, 2005, p. 47.
Figures number 2-5 from Batten et al, The Creation Answers Book, Creation Book Publishers, 2017, pp. 66-70.